The presence of sudden cardiac death
is estimated to occur 300,000 to 350,000 annually with over 90% of such deaths
as a results of ventricular fibrillation (VF). ACLS guidelines dictate that
after addressing reversible causes or factors leading to the arrhythmia
(hypoxia, electrolyte disturbances, mechanical factors, volume depletion),
defibrillation should be performed with 360 J for monophasic defibrillators or
120-200 J for biphasic defibrillators. In a subset of patients, however,
conventional means of terminating ventricular arrhythmias does not work. Energy
requirements for refractory VF is controversial and, recently, the idea of
double sequence defibrillation (DSD) has become a solution to refractory VF and
subsequent death.
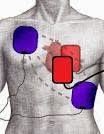
DSD is performed by attaching two sets of
defibrillation pads rather than one and delivering two shocks as near
simultaneously as possible, delivering electricity to the myocardial tissue in
parallel pathways. The idea is that several factors affect the defibrillation
threshold such as obesity, chronic lung disease, antiarrhythmic agents,
decreased ejection fraction, body position/habitus, and presence of implanted internal
defibrillator.
Hoch et al advocate for DSD in refractory VF.
Hoch found that all five patients in the study converted to normal sinus rhythm
after double sequence defibrillation at a total of 720 J. Other support for DSD
come from the Cabanas paper, a retrospective case series which looked at 10
cases of refractory VF. In the paper, DSD successfully terminated 70% of
refractory VF, attaining ROSC in 30% of those patients. Unfortunately, however,
none of these patients survived to discharge. A contributing factor to explain
the fact that there were no survivors to discharge was that DSD was performed
too late. In the cases reviewed, 6.5 single shocks were given prior to DSD and
in 6 of those cases, DSD was performed 35 minutes into resuscitation, which was
probably too late.
Anterior-Lateral/Anterior-Lateral
Anterior-Lateral/Anterior-Posterior
References
- Chang,
Mau-Song et al. Double and Triple Sequential Shocks Reduce Ventricular
Defibrillation Threshold in Dogs With and Without Myocardial Infarction.
Journal of the American College of Cardiology 1986; 8 (6): 1393-1405.
- Hoch,
David H et al. Double Sequence External Shocks for Refractory Ventricular
Fibrillation. JAC 1994; 23(5): 1141-1145.
- Zipes,
Douglas P et al. Management of Patients with Ventricular Arrhythmias and
the Prevention of Sudden Cardiac Death. American Heart Association,
American College of Cardiology Foundation 2006.
- Pantridge,
J. F et al. Electrical Requirements for Ventricular Defibrillation.
British Medical Journal 1975; 2: 313-315.
- Geddes,
L. A. et al. Electrical Dose of Ventricular Defibrillation of Large and
Small Animals Using Precordial Electrodes. Journal of Clinical
Investigation 1974; 53(1): 310-319.
- Adgey,
A. A. J. Electrical energy requirements for ventricular defibrillation.
British Heart Journal 1978; 40: 1197-1199.
- Cabaas,
J. G. Double sequence external defibrillation in out-of-hospital refractor
ventricular fibrillation: a report of ten cases. Prehospital Emergency
Care 2015; 19(1): 126-130.
- Tacher,
W. A. et al. Energy dosage for human trans-chest electrical ventricular
defibrillation. New England Journal of Medicine 1974; 290: 214-215